Answer : The correct option is, (A) Equilibrium shifts right to produce more product.
Explanation :
According to the Le Chatelier's principle, when we are adding or removing the products or reactants from the reaction, the equilibrium will shift towards that side where the less number of molecule.
The given equilibrium reaction is,
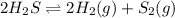
In the given reaction, when we are removing the hydrogen gas from the reaction then the equilibrium will shift towards the right side to produce more product.
Hence, the correct option is, (A)