Answer: The chemical equation is given below.
Step-by-step explanation:
We are given a equation that represents ionization of barium nitrate salt.
Ionization reaction is defined as the reaction in which a salt breaks into its respective ions. The chemical equation for the ionization of barium nitrate is given as:
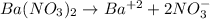
By Stoichiometry of the reaction:
1 mole of barium nitrate ionizes to produce 1 mole of barium ions and 2 moles of nitrate ions.
Hence, the chemical equation is given above.