D. Same energy level but different sublevel.
Step-by-step explanation
There are four quantum numbers [1]:
- n, the principal quantum number,
- l, the orbital angular momentum quantum number,
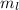 , the magnetic quantum number, and
, the magnetic quantum number, and
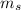 , the electron spin quantum number.
, the electron spin quantum number.
As their names might suggest:
- n determines the main energy level of an electron.
- l determines the type of sublevel of an electron.
- Each sublevel might contain more than one orbital.
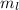 gives the orbital of an electron.
gives the orbital of an electron. - Each orbital contains up to two electrons.
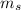 tells two electrons in the same orbital apart.
tells two electrons in the same orbital apart.
The two electrons in question come from the same atom. The question suggests that they have the same n,
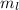 , and
, and
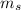 . As a result, both electrons are in main energy level n = 3. They share the same spin.
. As a result, both electrons are in main energy level n = 3. They share the same spin.
However, the two electrons differ in their value of l.
- l = 2 for the first electron. It belongs to a d sublevel.
- l = 1 for the second electron. It belongs to a p sublevel.
Reference
[1] Kamenko, Anastasiya, et. al, "Quantum Numbers", Physical & Theoretical Chemistry, Chemistry Libretexts, 24 Mar 2017.