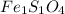Answer:
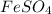
Solution : Given,
If percentage are given then we are taking total mass is 100 grams.
So, the mass of each element is equal to the percentage given.
Mass of Fe = 36.76 g
Mass of S = 21.11 g
Mass of O = 42.13 g
Step 1 : convert given masses into moles.
Moles of Fe =
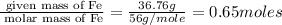
Moles of S = \frac{\text{ given mass of S}}{\text{ molar mass of S}}= \frac{21.11g}{32g/mole}=0.65moles
[/tex]
Moles of O = \frac{\text{ given mass of O}}{\text{ molar mass of O}}= \frac{42.13g}{16g/mole}=2.63[/tex]
Step 2 : For the mole ratio, divide each value of moles by the smallest number of moles calculated.
For Fe =
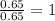
For S =
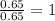
For O =
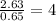
The ratio of Fe : S : O= 1 : 1 : 4
Hence the empirical formula is