- E(Bonds broken) = 1371 kJ/mol reaction
- E(Bonds formed) = 1852 kJ/mol reaction
- ΔH = -481 kJ/mol.
- The reaction is exothermic.
Step-by-step explanation
2 H-H + O=O → 2 H-O-H
There are two moles of H-H bonds and one mole of O=O bonds in one mole of reactants. All of them will break in the reaction. That will absorb
- E(Bonds broken) = 2 × 436 + 499 = 1371 kJ/mol reaction.
- ΔH(Breaking bonds) = +1371 kJ/mol
Each mole of the reaction will form two moles of water molecules. Each mole of H₂O molecules have two moles O-H bonds. Two moles of the molecule will have four moles of O-H bonds. Forming all those bond will release
- E(Bonds formed) = 2 × 2 × 463 = 1852 kJ/mol reaction.
- ΔH(Forming bonds) = - 1852 kJ/mol
Heat of the reaction:
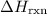 is negative. As a result, the reaction is exothermic.
is negative. As a result, the reaction is exothermic.