Answer:
2Li + F₂ → 2LiF
Step-by-step explanation:
The reaction expression is given as:
Li + F₂ → LiF
We are to balance the expression. In that case, the number of atoms on both sides of the expression must be the same.
Let use a mathematical approach to solve this problem;
Assign variables a,b and c as the coefficients that will balance the expression:
aLi + bF₂ → cLiF
Conserving Li: a = c
F: 2b = c
let a = 1, c = 1 and b =
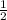
Multiply through by 2;
a = 2, b = 1 and c = 2
2Li + F₂ → 2LiF