Answer:
Mass of oxygen in glucose = 29.3g
Step-by-step explanation:
Mass of glucose given is 55grams.
We are to find the mass of oxygen in this compound.
In the compound we have 6 atoms of oxygen.
Solution
To find the mass of oxygen in glucose, we calculate the formula mass of glucose. We now divide the formula mass of the oxygen atom with that of the glucose and multiply by the given mass to find the unkown mass.
Atomic mass of C = 12g
H = 1g
O = 16g
Formula mass of C₆H₁₂O₆ = {(12x6) + (1x12) + (16x6)} = 180
Mass of O in glucose =
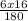 x 55
x 55
=
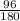 x 55
x 55
= 0.53 x 55
Mass of oxygen in glucose = 29.3g