Answer:
1) The value of the
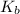 is 0.07742°C/m.
is 0.07742°C/m.
2) 0.261°C is the freezing-point depression of a solution.
Step-by-step explanation:
1)
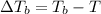
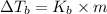
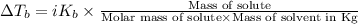
where,
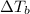 =Elevation in boiling point
=Elevation in boiling point
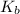 = boiling point constant of solvent= 3.63 °C/m
= boiling point constant of solvent= 3.63 °C/m
1 - van't Hoff factor (non-electrolyte solute)
m = molality
We have :
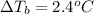
m = 3.1 m
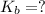
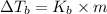
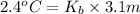
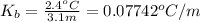
The value of the
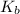 is 0.07742°C/m.
is 0.07742°C/m.
2)
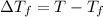
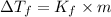

where,
 =depression in freezing point
=depression in freezing point
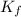 = freezing point constant of solvent= 1.86°C/m
= freezing point constant of solvent= 1.86°C/m
1 - van't Hoff factor (non-electrolyte solute)
m = molality
We have , Moles of solute = 0.705 mol
Mass of solvent = 5.02 kg
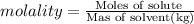
m =
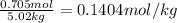
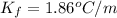
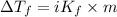
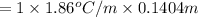
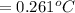
0.261°C is the freezing-point depression of a solution.