Answer : The pressure of the gas is, 14.925 atm
Solution :
using ideal gas equation,
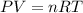
where,
n = number of moles of gas = 0.3 mole
P = pressure of the gas = ?
T = temperature of the gas =
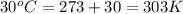
R = gas constant = 0.0821 Latm/moleK
V = volume of the gas = 0.5 L
Now put all the given values in the above equation, we get the pressure of the gas.
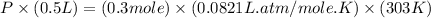
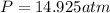
Therefore, the pressure of the gas is, 14.925 atm