Answer: Option (A) is the correct answer.
Step-by-step explanation:
The given equation is as follows.
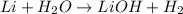
Correct orders of the steps Mason must have followed to balance the equation are as follows.
- Then Mason noticed that the two hydrogen (H) atoms on the reactant side and the three hydrogen atoms on the product side were not balanced.
- So, he added a coefficient of 2 in front of the water (H2O) reactant and the lithium hydroxide (LiOH) product in order to correct the imbalance as follows.
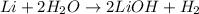
- Noticed the imbalance between the one lithium (Li) atom on the reactant side and the two lithium atoms on the product side.
- After that, he added a coefficient of 2 in front of the lithium (Li) reactant in order to correct the imbalance as follows.
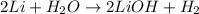
- Now, Mason observed that the equation was balanced.
Thus, we can conclude that D, E, C, A, B correctly orders the steps Mason must have followed to balance the equation
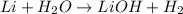 .
.