Answer: The mass of nitrogen dioxide for given number of moles are 158.7 grams.
Step-by-step explanation:
To calculate the mass for given number of moles, we use the equation:
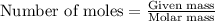
We are given:
Moles of nitrogen dioxide = 3.45 moles
Molar mass of nitrogen dioxide =
![[14+(2* 16)]=46g/mol](https://img.qammunity.org/2019/formulas/chemistry/middle-school/xd5s1t1i1jpi0dnod3b5240lv5pd4m508x.png)
Putting values in above equation, we get:
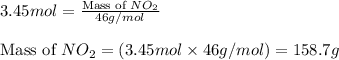
Hence, the mass of nitrogen dioxide for given number of moles are 158.7 grams.