Given :
A gas with a volume of 2 L at 25°C is placed into a container that is 4 L.
To Find :
The new temperature of the gas.
Solution :
Since, their is no information regarding pressure. We will assume that pressure is constant.
Now, we know when at constant pressure, volume is directly proportional to volume.
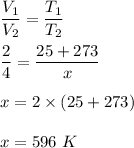
Therefore, the new temperature of the gas is 596 - 273 K = 323 K.