Answer : The correct option is, (B) Linear
Explanation :
Formula used :
![\text{Number of electrons}=(1)/(2)[V+N-C+A]](https://img.qammunity.org/2019/formulas/chemistry/middle-school/9092ozegyo6owf6gbmfgnwh1y8xvwkxj9r.png)
where,
V = number of valence electrons present in central atom
N = number of monovalent atoms bonded to central atom
C = charge of cation
A = charge of anion
Now we have to determine the hybridization of the
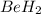 molecules.
molecules.
![\text{Number of electrons}=(1)/(2)* [2+2]=2](https://img.qammunity.org/2019/formulas/chemistry/high-school/ltv8jyfe59d82z1h4hq4k8nc52ugn6vmwl.png)
The number of electrons is 2 that means the hybridization will be
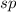 and the electronic geometry of the molecule will be linear.
and the electronic geometry of the molecule will be linear.
The three-dimensional shape of the molecule with the Lewis structure are shown below.