Answer : The volume of the gas at standard pressure (1 atm) is, 162.45 L
Solution : Given,
Initial pressure of gas = 650 mmHg =
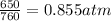
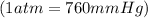
Initial volume of gas = 190 L
Final pressure of gas = 1 atm (standard pressure is 1 atm)
Formula used :
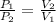
where,
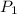 = initial pressure of gas
= initial pressure of gas
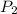 = final pressure of gas
= final pressure of gas
 = initial volume of gas
= initial volume of gas
 = final volume of gas
= final volume of gas
Now put all the given values in the above formula, we get
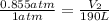
By rearranging the terms, we get the final volume of the gas.
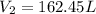
Therefore, the volume of the gas at standard pressure (1 atm) is, 162.45 L