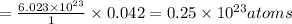Answer:
a)
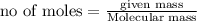
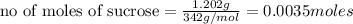
b) Moles of carbon in 1 mole of sucrose
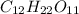 = 12 moles
= 12 moles
Moles of carbon in 0.0035 moles of sucrose
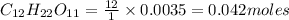
Moles of hydrogen in 1 mole of sucrose
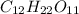 = 22 moles
= 22 moles
Moles of hydrogen in 0.0035 moles of sucrose
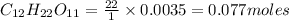
Moles of oxygen in 1 mole of sucrose
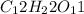 = 11 moles
= 11 moles
Moles of oxygen in 0.0035 moles of sucrose
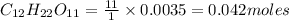
c) 1 mole of carbon contains
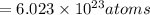
0.042 moles of carbon contain
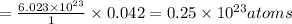
1 mole of hydrogen contains
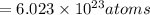
0.077 moles of hydrogen contain
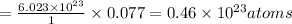
1 mole of oxygen contains
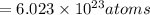
0.042 moles of oxygen contain