Answer : The mass of
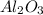 produced will be, 101.96 grams
produced will be, 101.96 grams
Explanation : Given,
Mass of Al = 54 g
Molar mass of Al = 27 g/mole
Molar mass of
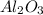 = 101.96 g/mole
= 101.96 g/mole
First we have to calculate the moles of Al.
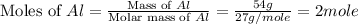
Now we have to calculate the moles of
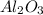
The given balanced chemical reaction is,
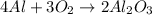
From the balanced reaction we conclude that,
As, 4 moles of Al react to give 2 moles of
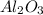
So, 2 moles of Al react to give
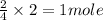 of
of
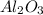
Now we have to calculate the mass of
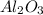
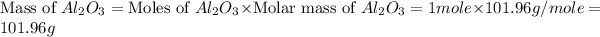
Therefore, the mass of
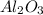 produced will be, 101.96 grams
produced will be, 101.96 grams