Answer: The given statement is false.
Step-by-step explanation:
Relation between kinetic energy and temperature is as follows.
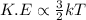
As, kinetic energy is directly proportional to temperature. So, when there will be increase in temperature then there will also occur increase in kinetic energy of the particles of a substance.
At high temperature, gases are desorbed, that is, they readily escape into the atmosphere.
Therefore, when gas particles move more slowly then it means kinetic energy of gas particles is very low. It also implies that temperature is low.
Hence, at low temperature only gases are absorbed.
Thus, we can conclude that the statement gases are absorbed more easily in hot water, is false.