Answer : The mass of of water present in the jar is, 298.79 g
Solution : Given,
Mass of barium nitrate = 27 g
The solubility of barium nitrate at
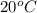 is 9.02 gram per 100 ml of water.
is 9.02 gram per 100 ml of water.
As, 9.02 gram of barium nitrate present in 100 ml of water
So, 27 gram of barium nitrate present in
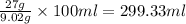 of water
of water
The volume of water is 299.33 ml.
As we know that the density of water at
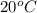 is 0.9982 g/ml
is 0.9982 g/ml
Now we have to calculate the mass of water.
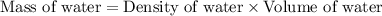
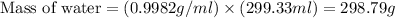
Therefore, the mass of of water present in the jar is, 298.79 g