Answer: There are
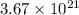 number of nicotine molecules in 1 gram of nicotine.
number of nicotine molecules in 1 gram of nicotine.
Step-by-step explanation:
NIcotine is an organic compound with chemical formula of
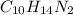
Given mass of nicotine = 1.00 g
Molecular mass of Nicotine = 162.23 g/mol
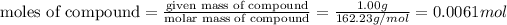
Number of molecules of compound:
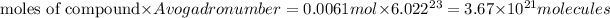
There are
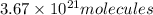 in 1 gram of nicotine.
in 1 gram of nicotine.