Answer:
B. Mg +
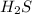 ⇒ MgS +
⇒ MgS +

Step-by-step explanation:
There are several types of chemical reactions depending on the reaction mechanisms: combination, single-displacement, double-displacement, combustion, decomposition, combustion and redox reactions.
In this case we have a single-displacement reaction, which means that a new chemical species (Mg) replaces other (H) in a given chemical compound (
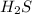 ). It happens because the electronegativity of H (a nonmetal) is greater than the Mg electronegativity. Thus H attracts the electrons strongly.
). It happens because the electronegativity of H (a nonmetal) is greater than the Mg electronegativity. Thus H attracts the electrons strongly.
That's why there is a transference of electrons from Mg to
 . We can say that Mg reduces
. We can say that Mg reduces
 .
.
Once
 is reduced to H, a covalent bond is formed between two hydrogen atoms, and then
is reduced to H, a covalent bond is formed between two hydrogen atoms, and then
 is released as a gas.
is released as a gas.