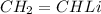Answer:
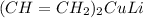
Step-by-step explanation:
Gilman reagent is also known as the Lithium organocuprates. They are used as nucleophiles in the organic synthesis.
Gilman reagent is used for C - C bond formation.
H H H
| | |
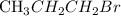 -----→ H - C -- C -- C -- C =
-----→ H - C -- C -- C -- C =
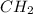
(1 bromo propane) | | | |
H H H H
(1 pentene)
Normally in C--C formation by Gilman reagent, the following reactions takes place :
R -- Br +
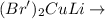 R--R' + R' Cu + Li Br
R--R' + R' Cu + Li Br
In 1 pentene, i.e.
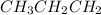

(from 1 bromo (from Gilman reagent)
propane)
So Gilman reagent is
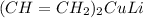
Organo lithium compound is