Answer:

Step-by-step explanation:
Hello!
In this case, since the formation of ammonia by starting with nitrogen and therefore hydrogen is:
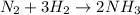
Which has an energy of reaction of:

We can compute the energy required for this reaction by first computing the moles of ammonia yielded by 21.7 grams of nitrogen (28.02 g/mol) via stoichiometry:

Thus, the energy turns out:

Best regards!