T = half life period of decay for atoms of element A = 20,000 years
N₀ = initial number of atoms of element A = 10,000 atoms
N = final number of atoms after time "t" = 2500 atoms
t = time of decay = ?
λ = decay constant = ?
decay constant is given as
λ = 0.693/T
λ = 0.693/20,000
λ = 0.00003465 years⁻¹
atoms after decay for time "t" is given as
N = N₀
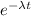
inserting the values
2500 = (10000)
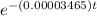
t = 40,000 years
so correct choice is
B) 40,000 years