Answer:
Lewis dot structure is the structure which represent the valence electrons of the element around the chemical symbol of the element.
Selenium is the 34th element of the periodic table. Atomic number of bromine is 34 that means it contains 34 electrons, Thus the electronic configuration is represented as:
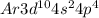
Thus the valence electrons are 6. Thus 6 electrons are represented around chemical symbol of Selenium i.e. Se.
Selenium can gain 2 electrons to attain stable configuration and thus oxidation number of Selenium is -2.