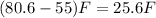Answer:
B) increase the room temp by 26°F
Step-by-step explanation:
From the given information:
Room temperature = 55 °F
Required temperature = 300 K
Conversion from K to °F:
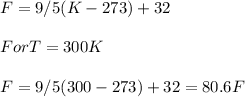
The required temperature in °F = 80.6°F
Therefore, the lab temperature must be increased by: