Step-by-step explanation:
When a reaction equation contains equal number of atoms on both reactant and product side then this type of chemical equation is known as balanced chemical equation.
For example, multiply this equation by 2 as follows.
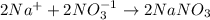
When sodium ions chemically combine with nitrate ions then it results into the formation of sodium nitrate.
Number of atoms on reactant side are as follows.
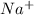 = 2
= 2
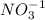 = 2
= 2
Number of atoms on product side.
Na = 2
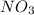 = 2
= 2
Therefore, as it contains same number of atoms on both reactant and product side.
Also, we can never change the subscripts present in a reaction equation but we can change the number of coefficients on each side in order to balance a chemical equation.