Answer:1) Volume of
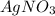 required is 55.98 mL.
required is 55.98 mL.
2) 0.62577 grams of
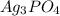 is produced.
is produced.
Step-by-step explanation:

1) Molarity of
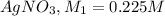
Volume of

Molarity of
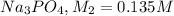
Volume of
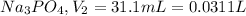
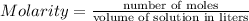
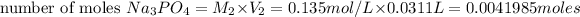
According to reaction, 1 mole of
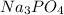 reacts with 3 mole of
reacts with 3 mole of
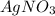 , then, 0.0041985 moles of
, then, 0.0041985 moles of
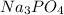 will react with:
will react with:
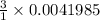 moles of
moles of
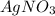 that is 0.0125955 moles.
that is 0.0125955 moles.
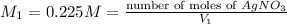
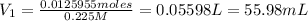
Volume of
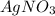 required is 55.98 mL.
required is 55.98 mL.
2)

Number of moles of
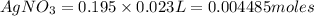
According to reaction, 3 moles of
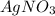 gives 1 mole of
gives 1 mole of
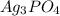 , then 0.004485 moles of
, then 0.004485 moles of
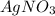 will give:
will give:
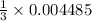 moles of
moles of
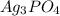 that is 0.001495 moles.
that is 0.001495 moles.
Mass of
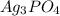 =
=
Moles of
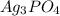 × Molar Mass of
× Molar Mass of
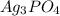
= 0.001495 moles × 418.58 g/mol = 0.62577 g
0.62577 grams of
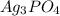 is produced.
is produced.