Answer:
 atoms
atoms
Step-by-step explanation:
According to avogadro's law, 1 mole of every substance contains avogadro's number
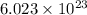 of particles and weighs equal to the molecular mass.
of particles and weighs equal to the molecular mass.
To calculate the moles, we use the equation:
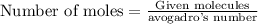
![[tex]\text{Number of moles of ammonia}=(18.06* 10^(23))/(6.023* 10^(23))=3moles](https://img.qammunity.org/2022/formulas/chemistry/high-school/2n2qitakf5d79n5ln02kv4bhm8pxsmuh05.png)
As 1 mole of
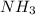 contains =
contains =
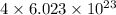 atoms
atoms
3 moles of
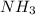 contains =
contains =
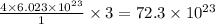 atoms
atoms
Thus
 atoms are present
atoms are present