Answer:
There are 15 grams of copper chloride in 50 cubic centimeters of solution.
Step-by-step explanation:
The statement is incorrect. The complete sentence will be presented below:
What is the mass of cooper chloride in 50 cubic centimeters of a 300 grams per cubic decimeters?
In Chemistry, a common unit for concentration is grams per cubic centimeter. A cubic decimeter equals 1000 cubic centimeters. First, we convert the given concentration into the requested unit:
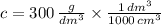
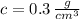
Which means there are 0.3 grams of copper chloride per each cubic centimeter of solution.
The amount of mass of copper chloride contained in 50 cubic centimeters of solution is calculated by dimensional analysis:
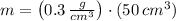
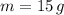
There are 15 grams of copper chloride in 50 cubic centimeters of solution.