Answer : Formation of yellow precipitate is the physical evidence that there has been a chemical reaction.
Explanation :
A chemical reaction happens when 2 or more substances chemically react with each other.
There are 4 evidences indicating that a chemical reaction has occurred which are mentioned below.
1. Change in color : On mixing, the reaction mixture can change color indicating that a chemical change may have occurred.
2. Formation of precipitate : A reaction in which a solid is formed on mixing 2 aqueous solutions is called as precipitation reaction. Here, the formation of solid indicates a chemical change.
ex.
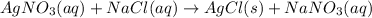
In above reaction, on mixing the solutions, a white precipitate of AgCl is formed which indicates a chemical change.
3. Evolution of gas : A chemical change also occurs when a gas is evolved on mixing 2 substances.
ex.
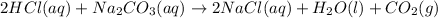
In above reaction, CO2 gas is evolved and bubbles can be seen in the form of effervescence.
4. Change in temperature : Certain chemical reactions absorb or evolve heat. When a chemical reaction evolves heat, the temperature of the final reaction mixture increases and when it absorbs heat, the temperature decreases. This increase and decrease in temperature indicates a chemical change.
ex.
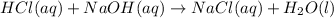
In above reaction, the final mixture becomes hot as the reaction releases heat. ( Exothermic)
Change in odor also indicates a chemical change.
For the given reaction, a yellow precipitate of PbI2 is getting formed.
Therefore formation of this precipitate is the physical evidence that there has been a chemical reaction.