Answer: A) Nonmetal carbon shares valence electrons with each nonmetal chlorine forming four covalent bonds.
Explanation:Carbon has atomic no 6 and has 4 valence electrons. It can only share electrons as it is difficult to gain or lose 4 electrons to complete it's octet.
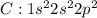
Chlorine has atomic no 17 and has 7 valence electrons and need one electron to complete its octet.
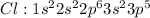
Thus carbon will share 4 electrons, one each with four chlorine atoms to form carbon tetra chloride.
Covalent bond is formed by sharing of electrons between atoms.
Ionic bond is formed by transfer of electrons between atoms.