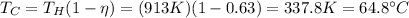Answer:
Step-by-step explanation:
The efficiency of a reversible heat engine is given by:
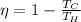
where
Tc is the cold temperature (outlet temperature)
Th is the hot temperature (temperature of the source)
In this problem, we know:
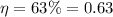
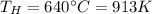
So, we can calculate the outlet temperature by re-arranging the formula: