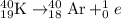Answer: Potassium-40 decays into argon gas over time.
Explanation: Potassium-argon dating is a dating method used to determine the age of sedimentary rocks by comparing the proportion of K-40 to Ar-40 in a sample of rock, and knowing the decay rate of K-40.
Potassium-40 undergoes decay following first order kinetics as given below: