Answer: 2 g
Explanation:
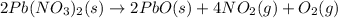
As can be seen from the balanced chemical equation, 2 moles of lead nitrate produce 4 moles of nitrogen dioxide.
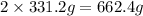 of lead nitrate produces
of lead nitrate produces
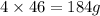 of nitrogen dioxide.
of nitrogen dioxide.
184 g of nitrogen dioxide will be produced by 662.4 g of lead nitrate
So 11.5 g of nitrogen dioxide will be produced by=
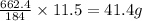 of lead nitrate
of lead nitrate
As can be seen from the balanced chemical equation, 2 moles of lead nitrate produce 1 mole of oxygen.
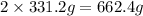 of lead nitrate produces 32 g of oxygen.
of lead nitrate produces 32 g of oxygen.
41.4 g of lead nitrate produces =
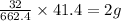 of oxygen.
of oxygen.