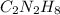Answer: The compound which is not an empirical formula is
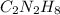
Step-by-step explanation:
Empirical formula is defined as the formula in which atoms in a compound are present in simplest whole number ratios.
For the given options:
Option A:
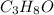
This compound is made by the combination of carbon, hydrogen and oxygen. The mole ratio of the elements are
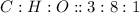
Option B:
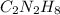
This compound is made by the combination of carbon, hydrogen and nitrogen. The mole ratio of the elements are
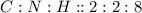
This ratio can be reduced to lowest numbers. The empirical formula of this becomes
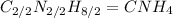
Option C:
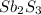
This compound is made by the combination of tin and sulfur atoms. The mole ratio of the elements are
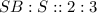
Option D:
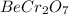
This compound is made by the combination of beryllium, chromium and oxygen. The mole ratio of the elements are
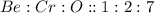
Hence, the compound which is not an empirical formula is