Answer:The average atomic mass of nitrogen is 14.004
Step-by-step explanation:
Abundance of nitrogen-14 = 99.6 %
Fractional abundance
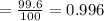
Abundance of nitrogen-15 = 0.4 %
Fractional abundance
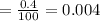
Average atomic mass of an element =
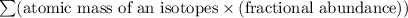
Average atomic mass of nitrogen =
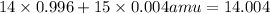
The approximate atomic mass of nitrogen is 14.004