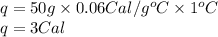Answer: 3 Calories of heat will be required by silver to raise the temperature to 1°C.
Explanation: Specific heat is defined as the heat required to raise the temperature by 1°C for 1 gram of substance.
Mathematically,
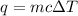
where,
q = heat required = ? Cal
m = Mass of the substance = 50 g
c = Specific heat of the substance =
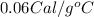
 = Change in the temperature = 1°C
= Change in the temperature = 1°C
Putting values in above equation, we get: