Answer:
12.3 years
Step-by-step explanation:
The equation of the radioactive decay can be written as follows:
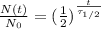 (1)
(1)
where
N(t) is the amount of radioactive sample left at time t
N0 is the amount of radioactive sample at time t=0
t is the time passed
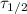 is the half-life of the isotope
is the half-life of the isotope
The problem tells us that after t=36.9 y, the amount of sample which has become stable is 7/8. This means that 7/8 of the sample has already decayed, so the amount of radioactive sample left is
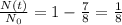
We can now re-arrange equation (1) by using this information and by substituting t=36.9 y we find:
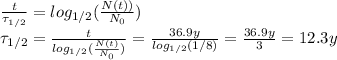
So, the answer is
12.3 years