Answer: CO is a limiting reagent with regards to the Fe production.
Step-by-step explanation:
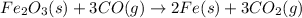
Moles of CO =
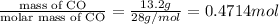
moles of
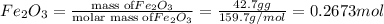
According to reaction , 3 mole of CO reacts with 1 mole of
 then , 0.4714 moles of CO will react with :
then , 0.4714 moles of CO will react with :
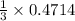 moles of
moles of
 that is 0.1571 moles.
that is 0.1571 moles.
0.4714 moles of CO will react with 0.1571 moles of
 which means that CO is present in limited amount acting as limiting reagent.
which means that CO is present in limited amount acting as limiting reagent.
Mole remaining of
 = 0.2673 mol - 0.1571 mol = 0.1102 mol
= 0.2673 mol - 0.1571 mol = 0.1102 mol
Hence, CO is a limiting reagent and
 is an excessive reagent.
is an excessive reagent.