Answer: 1. Option (A) is the correct answer.
2. Option (D) is the correct answer.
Step-by-step explanation:
When there is formation of an insoluble salt due to chemical reaction where two ions combine together in an aqueous solution then this insoluble salt is known as precipitate.
Therefore, chemical precipitation occurs when a product is insoluble and precipitates out of a solution.
Whereas reactants dissolve in precipitation reaction that is why a chemical reaction takes place in which an insoluble product is formed.
For example,
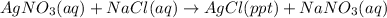
Here AgCl is the precipitate which is insoluble.
Thus, we can conclude the following.
- Chemical precipitation occurs when a product is insoluble and precipitates out of a solution.
- By comparison, precipitation reactions require the reactants to be soluble in the solvent.