Answer: The given element is non-metal and the number of valence electrons are 7
Step-by-step explanation:
There are 2 types of elements in the periodic table:
- Metals are the elements which have a tendency to loose electrons and thus they form cations. Example: Sodium will loose 1 electron to form
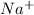 ion.
ion. - Non-metals are the elements which have a tendency to gain electrons and thus they form anions. Example: Chlorine will gain 1 electron to form
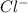 ion.
ion.
Valence electrons are defined as the electrons which are present in the outermost shell of an atom. Outermost shell has the highest value of 'n' that is principal quantum number.
We are given:
Fluorine is the 9th element of the periodic table having electronic configuration of
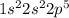
This element will gain 1 electron to form
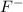 ion
ion
Number of valence electron in fluorine element = 2 + 5 = 7
Hence, the given element is non-metal and the number of valence electrons are 7