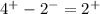Electron (Negative Charge) = -1
Nuetron (Nuetral Charge) = 0
Proton (Positive Charge) = 1
Because nuetrons are Nuetral we could have an infinite number and it wouldn't affect the charge.
Even infinity times zero is zero.
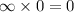
So, in most atoms the number or protons and nuetrons will be the same anyway.
I see 4 protons here and 4 nuetrons. Which ones are which I'm not sure but it doesn't matter because there is an equal amount. if there were 3 nuetrons and 4 protons we would be in trouble and unable to answer the problem without a label showing which is which.
4 protons on the presence of 2 electrons will balance to a charge of: