Answer: 1.94 kg
Explanation: Given :

Sample 1 produces 1.65 kg of Mg and 2.58 kg of
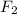 .
.
As every chemical reaction follows law of conservation of mass, which says that the mass of products must be equal to the mass of reactants.
Thus mass of
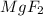 should be 1.65+2.58 kg = 4.23 kg
should be 1.65+2.58 kg = 4.23 kg
1.65 kg of Mg was produced by 4.23 kg of
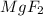
Now the second sample produces 1.24 kg of Mg.
1.24 kg of Mg will be produced by=
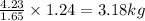
Now again law of conservation of mass is to be followed so mass of
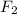 will be= mass of
will be= mass of
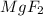 - mass of
- mass of
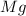
=(3.18 kg -1.24 kg) = 1.94 kg