6.69 g.
Step-by-step explanation
Make sure the chemical equation is balanced:
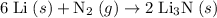
Assuming that one mole of N₂ is consumed. How many grams of each reactant will that take? Refer to a periodic table for data on atomic mass.
- 6 × 6.94 = 41.64 grams of Li;
- 1 × (2 × 14.01) = 28.02 grams of N₂.
4.00 grams of each reactant are available. How many moles of N₂ can they consume?
- 4.00 grams of Li will lead to up to 4.00 / 41.64 = 0.09606 moles of the reaction.
- 4.00 grams of N₂ will lead to up to 4.00 / 28.02 = 0.1428 moles of the reaction.
However, only 0.09606 moles of the reaction is possible, since Li would have ran out before all 4.00 grams of N₂ are used up.
Each mole of the reaction makes 2 moles of Li₃N. 0.09606 moles of the reaction will produce 0.09606 × 2 × (3 × 6.94 + 14.01) = 6.69 grams of Li₃N.