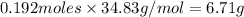Answer: 6.71 g
Step-by-step explanation:
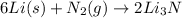
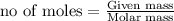
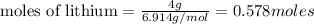
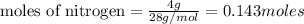
Limiting reagent is the reagent which limits the formation of product. Excess reagent is one which is in excess and thus remains unreacted.
Thus lithium is the limiting reagent and nitrogen is the excess reagent.
As can be seen from the balanced chemical equation, 6 moles of lithium reacts with 1 mole of nitrogen to give 2 moles of lithium nitride.
Thus 0.578 moles of lithium react with 0.096 moles of nitrogen.
6 moles of lithium give = 2 moles of lithium nitride
Thus 0.578 moles of lithium give=
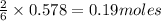 of lithium nitride.
of lithium nitride.
Mass of lithium nitride
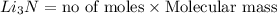
Mass of lithium nitride
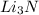 =
=