Answer:
32 electrons are the maximum electrons found in the N shell.
Step-by-step explanation:
According to the periodic table, Maximum number of electrons in any energy level of known element is 32 which is found in N shell.
Each shell can contain fixed number of electrons. The first shell is the K shell that holds two electrons, the second shell that is L shell which holds eight electrons(2+6), The third shell is M shell which holds 18 electrons(2+6+10), and the fourth shell is N shell which holds the maximum number of electrons that is thirty two(2+6+10+14) electrons. The general formula is
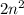 electrons.
electrons.
The outermost shell is called the valence shell and the electrons present in these shell are called valence electrons. Each shell consists of one or more subshells, and each subshell consists of one or more atomic orbitals.