T = half life of cobalt-60 = 5.3 years
λ = decay constant for cobalt-60 = ?
decay constant is given as
λ = 0.693/T
inserting the values
λ = 0.693/5.3 = 0.131
N₀ = initial amount of cobalt-60 = 10 g
t = time of decay = 21.2 years
N = final amount of cobalt-60 after time "t"
final amount of cobalt-60 after time "t" is given as
N = N₀
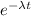
inserting the values
N = 10
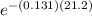
N = 10
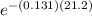
N = 10 x 0.0622 g
N = 0.63 g
hence
C. 0.63 g