Answer:
T_final = 279.4 [°C]
Step-by-step explanation:
In order to solve this problem, we must use the following equation of thermal energy.
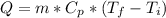
where:
Q = heat = 9457 [cal]
m = mass = 79 [g] = 0.079 [kg]
Cp = specific heat = 0.5 [cal/g*°C]
T_initial = initial temperature = 40 [°C]
T_final = final temperature [°C]
![9457 = 79*0.5*(T_(f)-40)\\239.41=T_(f)-40\\\\T_(f)=279.4[C]](https://img.qammunity.org/2022/formulas/physics/high-school/tgatnfjb1zg6m71rqkyojpeafviijew0ii.png)