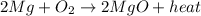Answer:
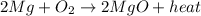 (2 atoms on the product side)
(2 atoms on the product side)
Step-by-step explanation:
Due to the law of conservation of mass, in every chemical reaction the number of atoms of each element on the reactant side must be equal to the number of atoms of the same element on the product side.
In this case, on the product side we have magnesium (which has two valence electrons) and oxygen (which has two vacancies in its outer shell). This means that they combine in a ratio 1:1, so the product of the reaction is
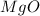
However, we must put a coefficient 2 in front of it in order to balance the number of atoms of each element: in fact, on the reactant side we have 2 atoms of magnesium and 2 of oxygen, so we must have two atoms of magnesium and 2 of oxygen on the product side as well. Therefore, the complete reaction is