Answer:
Q = 1190 [cal]
Step-by-step explanation:
In order to solve this problem, we must use the following equation of thermal energy.
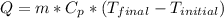
where:
Q = heat energy [cal]
Cp = specific heat = 1 [cal/g*°C]
T_final = final temperature = 120 [°C]
T_initial = initial temperature = 50 [°C]
m = mass of the substance = 17 [g]
Now replacing:
![Q=17*1*(120-50)\\Q=1190[cal]](https://img.qammunity.org/2022/formulas/physics/high-school/tpx94z8k8txmy2qql5cn2nfhp37t6h1fbd.png)